Caffeine
Publié le 22/05/2020

Extrait du document
«
EXTRACTION OF CAFFEINE AND PROPERTIES OF CAFFEINE
MOLECULE
What’s caffeine (C
8 H
10 N
4 O
2 )?
Caffeine is a member of the class of compounds called alkaloids.
Alkaloids are nitrogen-
containing basic compounds that are found in plants.
They usually taste bitter and are often
physiologically active in humans.
The common names for some
of these compounds are probably familiar, even if their structures
aren’t: nicotine, morphine, strychnine and cocaine.
In some cases
they may act as pesticides.
Caffeine is found in a number of
things that people ingest.
Caffeine acts as a stimulant, stimulating
the heart, respiration and the central nervous system.
It is also a
diuretic.
Ingesting caffeine generally produces a sensation of
heightened alertness, but not necessarily well-being.
Coffee 80 – 125 mg per cup
Coffee, decaffeinated 2 – 4 mg per cup
Tea 30 – 75 mg per cup
Cocoa 5 – 40 mg per cup
Milk Chocolate 6 mg per ounce
Baking Chocolate 35 mg per ounce
Properties of caffeine :
Many people rely on it as their morning’s “go-juice”.
Commonly found in coffee, tea, and
cola, it is readily available for consumption.
It is physically addictive; a person who drinks as
few as four (4) cups of coffee per day and tries to stop may experience headache, insomnia
and possibly nausea as symptoms of withdrawal.
For an average adult, a lethal dose is around
ten grams of caffeine (according to the MSDS).
For children (or smaller adults), this value is
less.
Thankfully, one or two cups of coffee won’t put you anywhere near that limit.
On the
other hand, eating the raw powder is a very bad idea.
Caffeine is a white powder.
It is water soluble (1 gram in 46 mL, or 21.7 grams per liter),
which is why we can drink it so redaily.
Caffeine will melt at a temperature of 238 Celsius
(460 Fahrenheit).
Caffeine is a mild diuretic, which means that it works to dehydrate your
body.
It is also slightly acidic in water, though the compound itself tastes bitter.
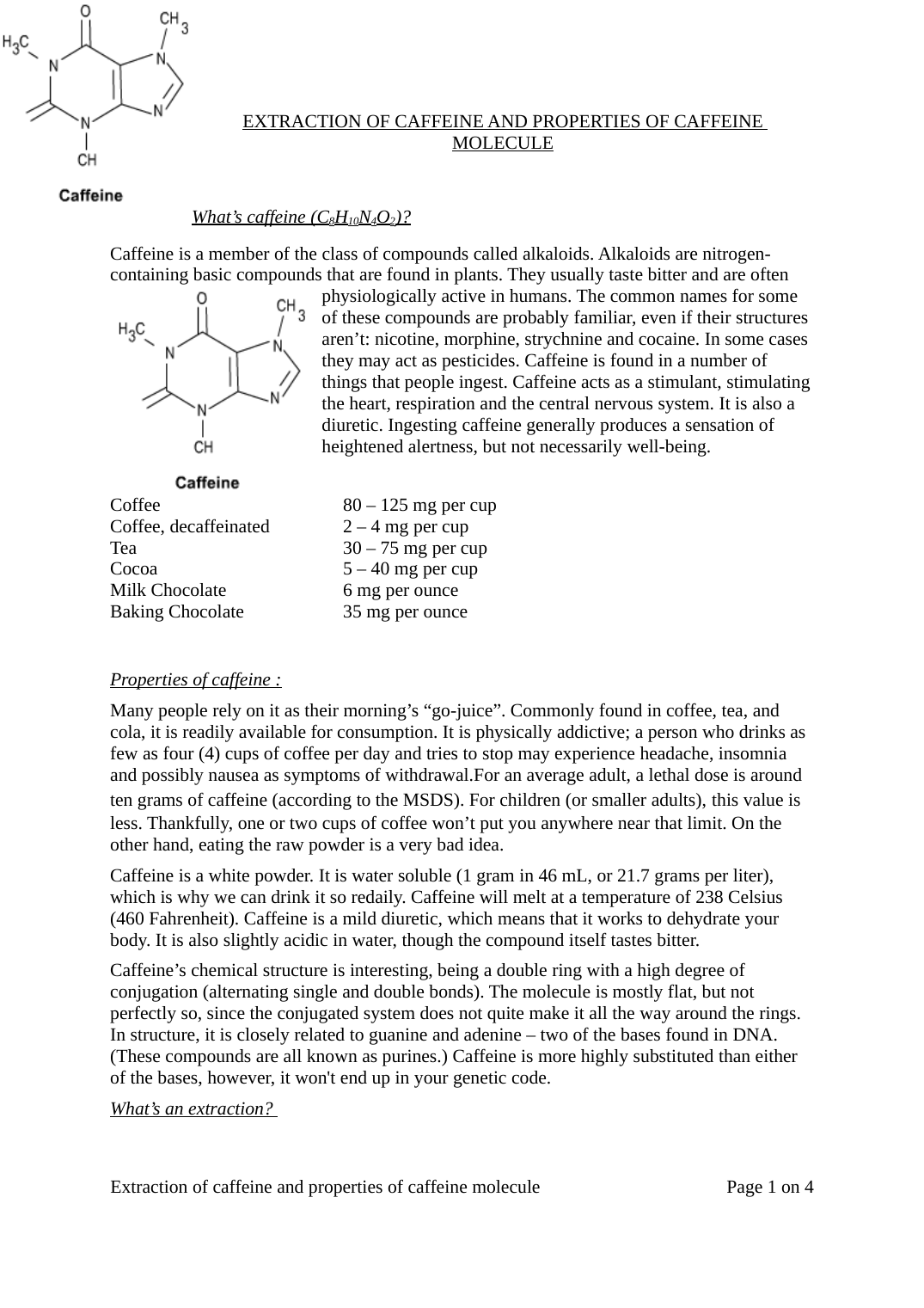
Caffeine’s chemical structure is interesting, being a double ring with a high degree of
conjugation (alternating single and double bonds).
The molecule is mostly flat, but not
perfectly so, since the conjugated system does not quite make it all the way around the rings.
In structure, it is closely related to guanine and adenine – two of the bases found in DNA.
(These compounds are all known as purines.) Caffeine is more highly substituted than either
of the bases, however, it won't end up in your genetic code.
What’s an extraction?
Extraction of caffeine and properties of caffeine molecule Page 1 on 4.
»
↓↓↓ APERÇU DU DOCUMENT ↓↓↓